A few weeks ago, we mentioned the promise of cellulose in medical devices due it being both biocompatible and a relatively accessible material. However, in this post we will take a closer look at the properties of cellulose that make it an ideal nanomaterial to consider, in particular how hydrogen bonding makes cellulose a very special molecule.
The orientation of glucose molecules in the chain causes the difference in strength between cellulose and starch, a strength that results from hydrogen bonding between adjacent strands of cellulose. Hydrogen bonds are very strong, which is why cellulose is an excellent fiber that is used from paper to hemp rope. The next time, you have a sheet of paper, try to pull it apart from the sides and you will notice how hard it is to do so.
Hydrogen bonding between cellulose chains also means that cellulose will not dissolve in water. Such a property both practical and useful. What this means in terms of using a medical device made of cellulose is that the medical device will not spontaneously dissolve after coming in contact with the water present in the human body. To explain the dissolving of cellulose in water in terms of free energy, the change in free energy of the reaction would be positive as the reaction is never spontaneous. This also tells us that the enthalpy change of the reaction is positive and the entropy change of the reaction is negative. If you do not understand how one would arrive at the previous conclusion, consider the Free Energy equation:
where G is Gibbs Free Energy, H is enthalpy, S is entropy, and T is temperature.
Hopefully this post gave you greater insight about how cellulose serves as such a great nanomaterial.
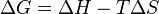
No comments:
Post a Comment