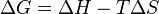Now, we know a lot more about spider silk, after a team of scientists was able to measure the elastic properties of an intact spider web and this increased knowledge should help with further innovations about silk that we previously mentioned in our blog here and here.
The Stanford researchers utilized a technique known as Billouin spectroscopy, which shines a laser at the spider web and then records the scattering of light to measure the mechanical properties of spider silk. In short, it is a much more complicated form of the spectrophotometry done in freshman and sophomore labs to measure absorbance values, except Billouin spectroscopy measure the mechanical properties of a material.
 |
| Measuring mechanical properties of spider silk using Billouin spectroscopy |
The researchers learned that although spider webs are made of uniform spider silk, the stiffness and elasticity of the silk varies between individual strands. They also discovered that the silk stiffens in conditions of 100% humidity to produce a tighter web, a behavior known as supercontraction. The second discovery that adjusting water content to alter the mechanical properties of spider silk is especially interesting and Kristie Koski, the lead researcher, say could lead to new and exciting advancements.